Complying Medical Devices to IEC 60645 Standard
The International Electrotechnical Commission (IEC) prepares and publishes international standards for all electrical, electronic, and related technologies. Any device designed, developed, and used as a medical device for audiometry/acoustics should comply with the requirements of the IEC 60645 standard.
IEC 60645 is a standard with different parts addressing a specific set of audio devices and their compliance.
Below are the applicable parts that are in scope for this case study:
- IEC 60645-1: Electroacoustics - Audiometric equipment - Part 1: Equipment for pure-tone and speech audiometry
- IEC 60645-3: Electroacoustics - Audiometric equipment - Part 3: Test signals of short duration
- IEC 60645-6: Electroacoustics - Audiometric equipment - Part 6: Instruments for the measurement of otoacoustic emissions
- IEC 60645-7: Electroacoustics - Audiometric equipment - Part 7: Instruments for the measurement of auditory brainstem responses.
Our customer is a leading manufacturer and service provider that offers medical devices and the relevant accessories and software, as well as services for diagnosis, monitoring and treatment of impairments and disorders that affect new-borns, the brain, nerves, balance, and hearing. The client’s medical devices, like the new-born hearing screener, are used in hospitals, medical clinics, and laboratories in many different regions.
The customer's new-born hearing screener is a portable and lightweight alternative to larger cart-based systems with different specifications. It is a handheld device that combines an otoacoustic emissions (OAE) screener with an auditory brainstem response (ABR) screener. It is used both during in-hospital screening and at non-hospital births and screening sites.
Business need
The client needed to prepare, implement, and update its existing audiometric device requirements and test cases according to the IEC 60645 standard for new-born hearing screener.
They also needed to update the requirements and develop test cases according to the IEC standards within a specific time frame. Failure to do so would have resulted in non-conformity, impacting sales and the credibility of the product.
Solution Implemented
We studied the IEC standards and identified relevant clauses for the product in question. We then developed a sophisticated checklist to map and draft the requirements. The checklist was presented to a technical team comprising product SME, labelling SME, hardware, firmware, and software design teams to review and confirm the applicable IEC standard clauses. Later we used the checklist to develop verifiable test cases for each finalised requirement.
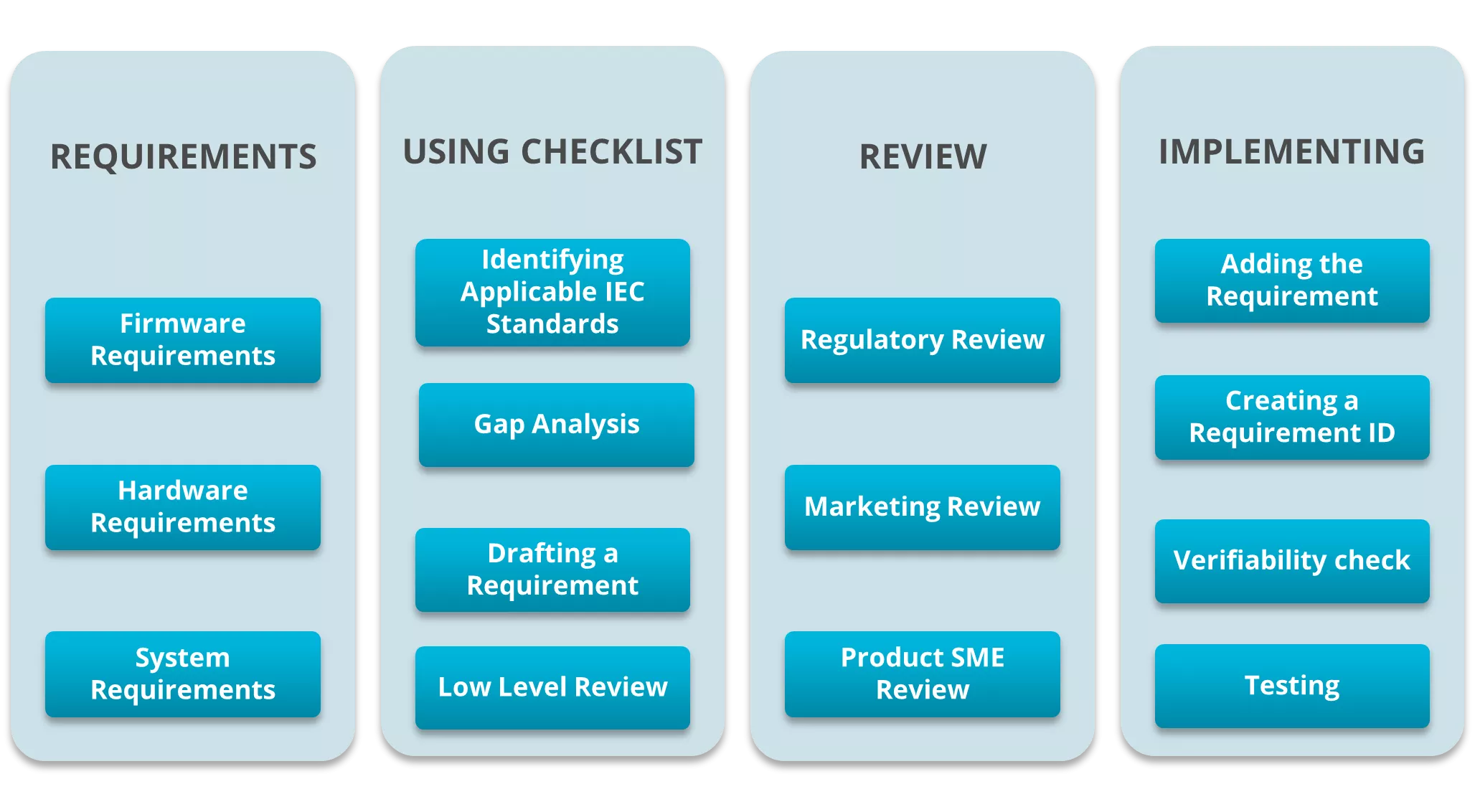
Verifying each requirement was the next step. This involved considering the testability of the requirements and developing rationale for the non-testable requirements. We also delegated testable requirements to the respective labs and test facilities involved. We drafted the process and divided it into the 4 sections shown in the figure below, to keep track of the status of every section. This allowed us to check the verifiability of a requirement at the end of each section.
Benefits Derived
Some of the key benefits achieved, are as follows:
- Client met the compliance requirements within the stipulated time frame and avoided non-conformity. It helped in continued sale of the devices.
- Established process/approach could be reused in similar situation, resulting in substantial time saving
Decos provides services in the area of product standardisation to ensure regulatory compliance for all kinds of requirements such as firmware, and user and product requirements, etc.
Contact Decos today to explore how our expertise in product standardization can elevate your offerings, ensuring regulatory compliance and bolstering your product's reputation in the market.
Discover more

Consequences of Excluding Human Factors Engineering from Medical Device Development


